

Training your brain to convert between 2D drawings and 3D bond placement in space is challenging. In both the space-filling and ball and stick models, the white spheres represent hydrogen atoms, and the black sphere represents the carbon atom. Click on the image to be taken to an interactive model for methane. All three images represent the same bond placements in space ( Figure 2). The dash and wedge notation is shown again below (left) adjacent to a space-filling model of CH 4 (center) and the ball and stick model (right). Notice that it is not pointing straight to the left, but it is slightly below the horizontal position. The fourth carbon-hydrogen bond is going back behind the H-C-H plane away from the observer. Notice that it is not pointing straight down, but it is slightly to the right of the center. Heading counter-clockwise on the H-C-H plane, the next bond is the solid wedge that represents the carbon-hydrogen bond coming out of the H-C-H plane towards the observer. These three atoms define an H-C-H plane that is located in the same plane as the paper/screen. We’ll talk below about why that angle is important to communicate the correct shape of methane. The second carbon-hydrogen bond in the plane of the paper is specifically drawn at ~109° from that first bond. Notice that on the structure with dash and wedge notation, one carbon-hydrogen bond points straight up from the carbon and is in the plane of the paper (indicated by the simple line). It communicates the structure of methane more clearly than a traditional Lewis structure without dash and wedge notation The correct Lewis structure with dash and wedge notation for CH 4 is depicted on the right in Figure 1. Bonds drawn with a solid triangle, or wedge, represent bonded atoms coming out of the page/screen towards the observer bonds drawn with dashed triangle, or dash, represent bonded atoms going into the page/screen away from the observer. Bonds drawn as simple lines are located in the plane of the page or screen. Chemists use dash and wedge notation to draw 3D molecules. Representing three dimensions on 2D paper (or computer screen) is challenging. The Lewis structure is misleading because it depicts methane as a planar ‘plus-shaped’ molecule, yet we know that methane is a non-planar, tetrahedral molecule.
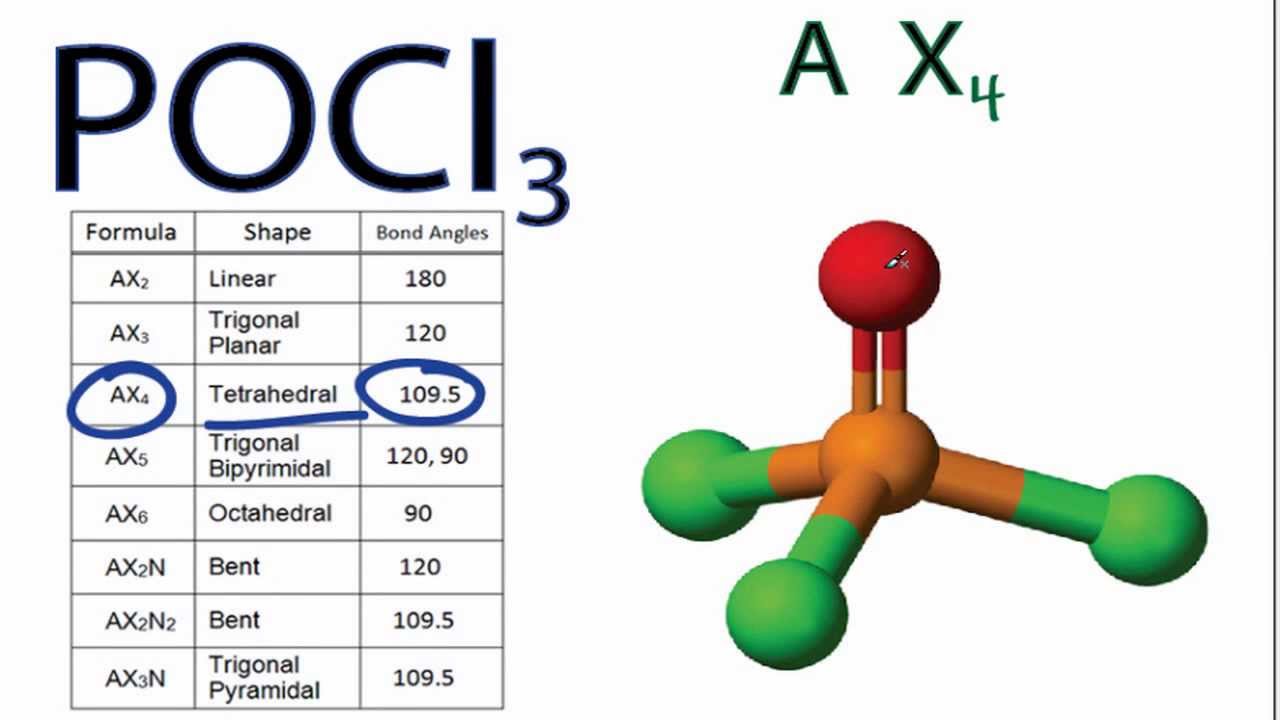
Methane (CH 4) depicted by a Lewis structure (left) and dash and wedge notation (right). Depicted on the left in Figure 1 is the Lewis structure for methane (CH 4) showing the central carbon atom singly bonded to four hydrogen atoms: Figure 1. The Lewis structure gives us meaningful information about the bonds between atoms, but Lewis structures do not depict how the molecule exists in three-dimensions. | Key Concepts and Summary | Glossary | End of Section Exercises | Lewis Structures with Wedge-Dash Notation | Lewis Structures with Wedge-Dash Notations | Draw and interpret 3-dimensional representations of molecules using “dashed” and “wedge” bonds and estimate bond angles.Molecular Geometry | Predicting Electron-pair Geometry and Molecular Geometry | VSEPR Review Chart | | VSEPR Theory | Electron-pair Geometry vs. Predict molecular shape as determined by Valence Shell Electron Pair Repulsion Theory (VSEPR).


 0 kommentar(er)
0 kommentar(er)
